At HemoShear we are driven to apply our scientific insights to develop medicines that improve the lives of patients with rare diseases. We are committed to understanding and helping meet the needs of patients and their families.
We are developing a treatment for methylmalonic acidemia (MMA) and propionic acidemia (PA). These ultra-rare genetic diseases are caused by inherited metabolic defects that result in the build-up of harmful toxins that can lead to severe organ damage, seizures, developmental deficits, and premature death. Although usually diagnosed soon after birth, there are currently no medical treatments for MMA or PA that improve quality of life or extend lifespan. Patients are treated by managing symptoms, following very restrictive diets, and sometimes receiving a liver and/or kidney transplant.
HemoShear has developed an experimental drug treatment for MMA and PA called HST5040. Our laboratory research shows that HST5040 has the potential to reduce toxins that impact the entire body. The drug is designed to be conveniently administered daily at home as a liquid taken either orally or through a gastric feeding tube.
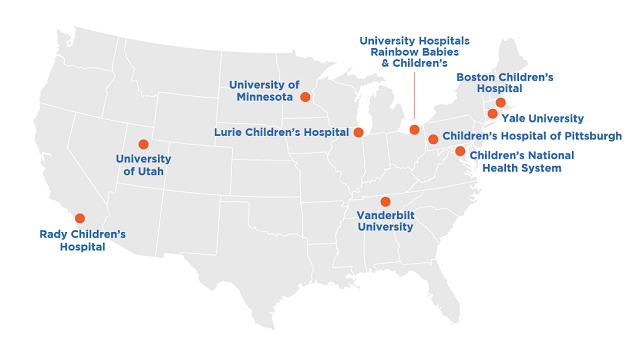
HemoShear is conducting the HERO (HElp Reduce Organic Acids) clinical study to assess our experimental drug HST5040 in patients with MMA and PA. HERO is being conducted with patients aged 2 years and older at select research centers across the United States.
Information about HERO can be found on the study website or on Clinicaltrial.gov. If there is a study site located near you, then you can speak with your physician or contact the site about participating. Please sign up if you would like to receive information from HemoShear about our clinical trial or other company developments.
The clinical study is being conducted at many centers in the United States. If there is a study site located near you, then you can speak with your physician or find site contact information on Clinicaltrial.gov.
HemoShear’s Chief Medical Officer Patrick Horn, MD, PhD, joined patient advocate Bryan Kelly for a DNA Today podcast interview on MMA and PA. Bryan is a great inspiration to patients and caregivers alike, using yoga and meditation techniques for pain relief and relaxation. Dr. Horn speaks about the science behind MMA and PA, current diagnosis methods and how these diseases are mostly managed by diet with many patients having ongoing challenges. He explains the HERO clinical trial. You can watch/listen to the episode here.
HemoShear, the Organic Acidemia Association and the Propionic Acidemia Foundation hosted a webinar in April 2021 for MMA and PA patients and caregivers. Watch this video to hear from the experts about current treatment approaches for MMA and PA, how the clinical research process works, how experimental oral drug HST5040 was developed and the design of our HERO (HElp Reduce Organic Acids) clinical trial.
If you have any questions regarding this policy, please contact: patients@hemoshear.com.
We expect to respond to inquiries within 7 business days.
HemoShear Therapeutics is committed to developing therapies that improve the lives of patients with rare genetic disorders with high unmet need, such as methylmalonic acidemia (MMA) and propionic acidemia (PA). We are working with a sense of urgency as we focus on designing and conducting clinical trials to assess safety and efficacy of our oral drug candidate HST5040 for patients with MMA and PA.
We are dedicated to expeditiously developing potential new therapies and believe that the most effective strategy for making them widely available to patients around the world is to maintain our focus on conducting the necessary research and development, including efficient enrollment and completion of the clinical trials required to support global regulatory approvals.
Clinical trials are required to establish whether an investigational medical product is safe and effective, and participants in clinical trials play a critical role in informing the understanding and treatment of serious diseases. We believe that participation in one of our clinical trials is the most appropriate way to access investigational drugs like HST5040 and we are not currently providing the drug for expanded access.
We would like to keep patients and families informed of our progress. Please sign up if you would like to receive information via email from HemoShear about our clinical trial or other company developments.
If you have any questions, please email us at patients@hemoshear.com
HemoShear Therapeutics, Inc.
501 Locust Avenue
Suite 301
Charlottesville, Virginia 22902